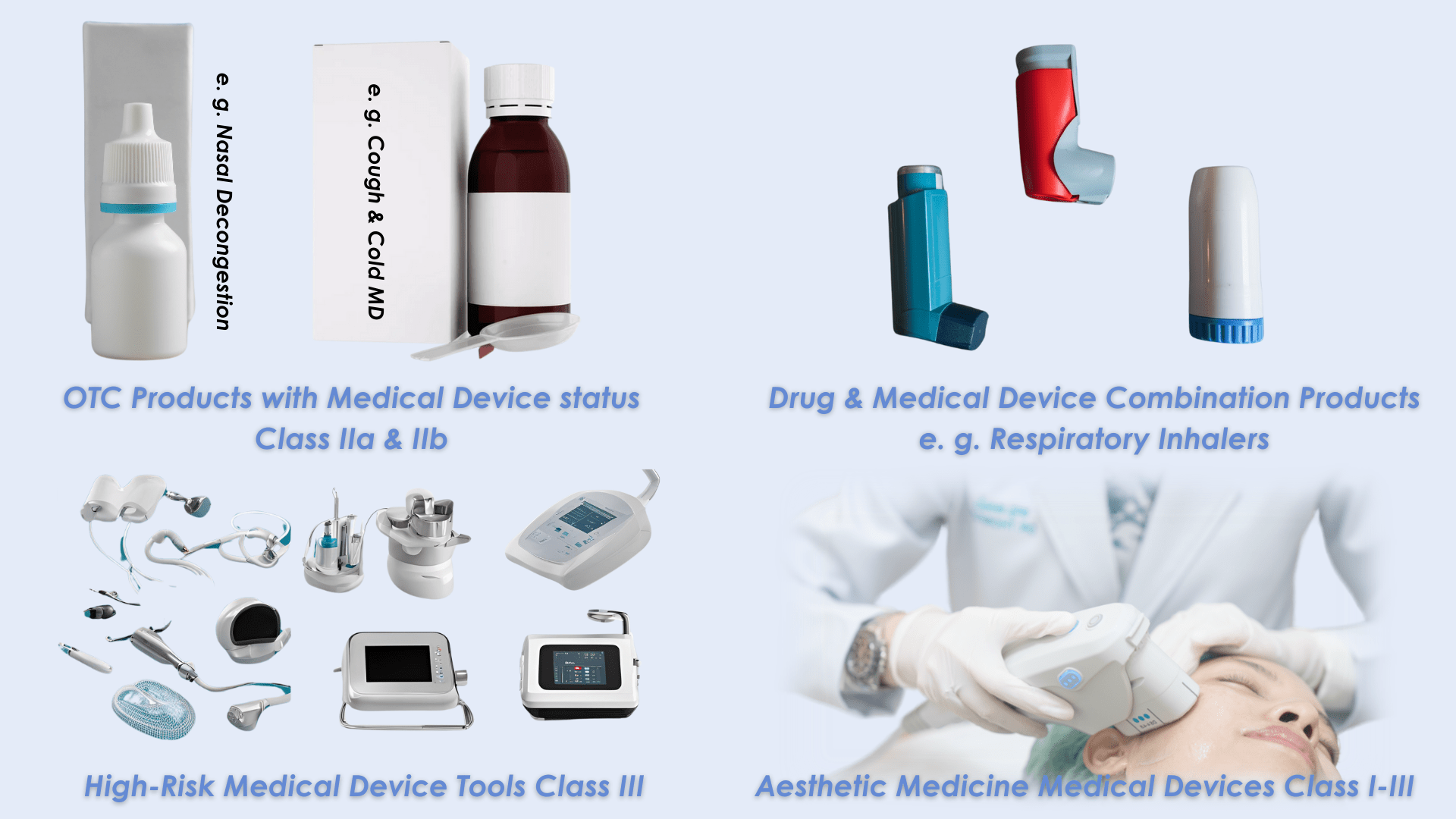
Image attributed to CPC Group, AI-generated image created using Canva Pro
Germany’s Medical Device and MedTech sector remains a powerhouse, with its market expected to grow at a solid CAGR of 15.8% until 2030. However, despite its success outlook, the European market faces stagnation, fragmentation, and mounting challenges like energy price surges due to geopolitical instability, compounded by a lack of cohesive industrial policies. To stay competitive and safeguard growth, Medical Device companies must diversify their portfolios—expanding beyond Europe is no longer just a strategy, but a necessity to thrive forward revenues and not be too dependent on the EU market.
German MedTech Market Insights and Global Diversification
European medical device companies, renowned for their advanced engineering and compliance with strict quality standards, are uniquely positioned to cater to the growing demand for innovative medical technologies in emerging economies. Germany, for example, is a leader in MD, with over 1,400 Medical Device companies. It ranks 2nd in Europe for medical device exports, generating a turnover of approximately €34 billion annually. But while recognized globally for its advanced technology and high-quality products, its industry operates within a challenging domestic and European landscape.
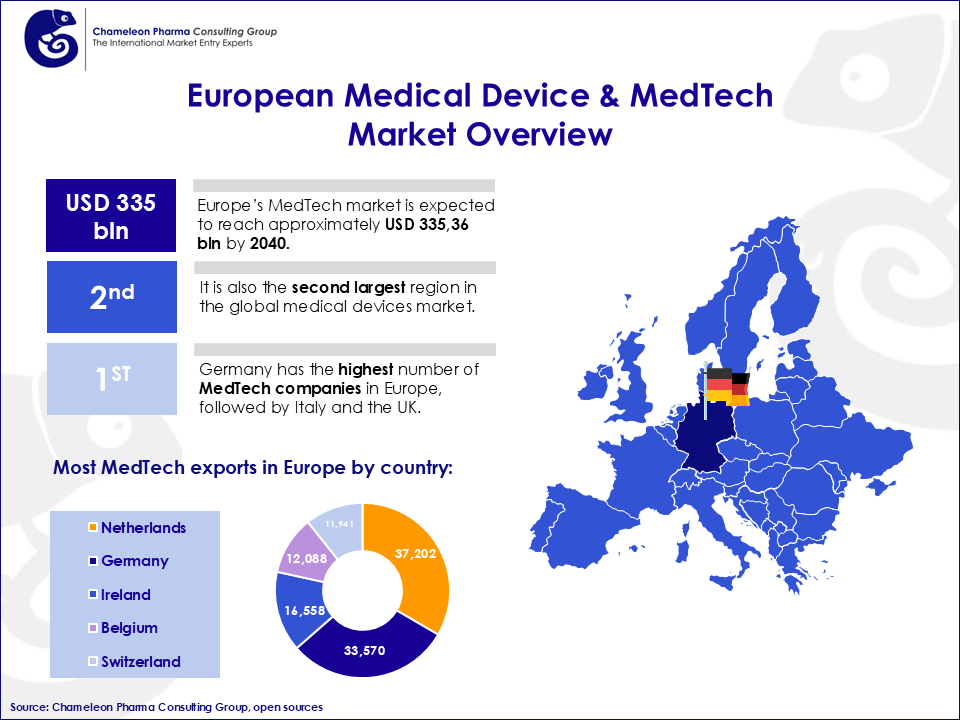
Figure 1. European Medical Device & MedTech Market Overview
Although the European MD market is the second-largest in the world, it can also be characterized by low growth compared to the dynamic opportunities available globally. Rising energy costs for Medical Devices class I, IIa, IIb and III can further hinder growth potential. This underscores the critical need for international market diversification. The market’s regulatory shifts and economic fluctuations underline the importance of tapping into high-growth markets outside the region.
Market Potential for Medical Device and MedTech Companies in Emerging Regions
Emerging markets in Latin America, Asia, and MENA are experiencing rapid growth, driven by increasing healthcare investments, and are hungry for new medical technologies. These regions not only promise higher growth rates but also provide a strategic avenue to reduce reliance on the European market, where economic and regulatory challenges persist.

Figure 2. Market Potential in Emerging Regions
Latin America: The region’s MedTech market is projected to grow at a CAGR of 8.21%, reaching USD 71.17 billion by 2035.
Key opportunities include:
- Regulatory Alignment: Colombia and Mexico are aligning closely with EU MDR standards, easing regulatory barriers for EU companies.
- Manufacturing Hubs: Puerto Rico remains a strategic hub for MedTech manufacturing due to its established infrastructure and skilled workforce, serving as a gateway to the Americas.
- Rising demand: Brazil, as the largest Pharma/OTC/MD in Latin America, presents a significant opportunity due to its vast market potential and growing Pharma sector. However, the country’s regulatory processes remain challenging. Partnering with experienced advisors such as CPC can help navigate these complexities and ensure successful market entry.
Asia: Asia is one of the top European MedTech export destinations, driven by its aging population and increasing healthcare expenditure. The region is poised to become the fastest-growing MedTech market globally, exceeding USD 180 billion by 2025 reaching a CAGR of 7.3% in 2030. Key drivers include:
- Government Investments: Nations like China and India are heavily investing in healthcare infrastructure and digital health technologies.
- Rapid Growth: Asia’s expanding middle class and rising demand for advanced medical devices present a lucrative opportunity.
MENA: Expected to reach a CAGR of around 6.2% by 2030, the MedTech market in MENA is poised for significant growth, with GCC countries leading the charge.
- Government Support: Policies and funding aimed at improving healthcare access and quality.
- Infrastructure Development: Expansion of hospitals and medical facilities in countries like the UAE and Saudi Arabia.
Strategic Approaches for Market Entry in LATAM, Asia, and MENA
The emerging markets present untapped opportunities where the “Made in Germany” label commands premium pricing due to its association with quality and innovation. By establishing a presence in these regions, companies can significantly reduce sales dependence on the EU and German markets, creating a more balanced global footprint.
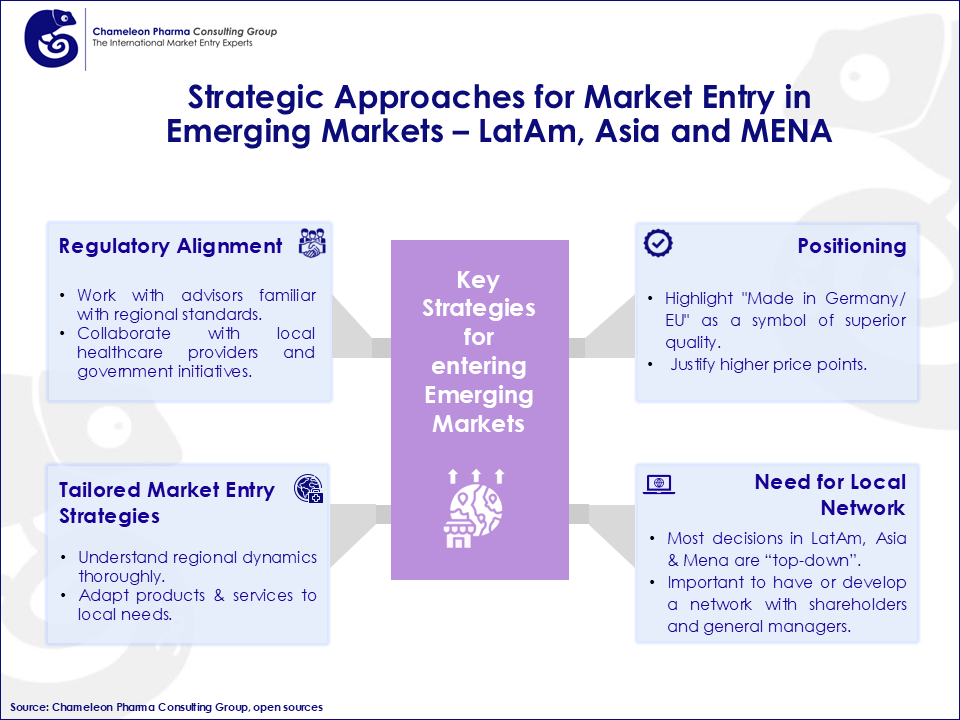
Figure 3. Strategic Approaches for Market Entry in Emerging Markets – LatAm, Asia and MENA
Expanding into Latin America, Asia, and MENA is a game-changing opportunity and a golden ticket to market leadership. This not only diversifies new revenue streams but also positions German and EU MedTech companies as global leaders. The strong reputation of EU MDR standards and the European product quality provides a competitive edge in these high-growth markets. By implementing tailored strategies and leveraging local partnerships, companies can outpace competitors and achieve sustainable growth while maintaining their commitment to innovation and quality.
Chameleon Pharma Consulting Group has more than 20 years of experience in providing support to Pharma, OTC, Medical Device, Aesthetic Medicine, looking to enter or expand in international aesthetic and pharma markets across promising regions like Latin America, Europe, Asia, the US/Canada, the Middle East, and CEE/CIS. With the expertise amassed from more than 300 international projects and 22 experts around the world, CPC Group offers:
- Systematic product and country analysis;
- Systematic local partner identification;
- Market Reports;
- Regulatory and registration assistance.
For your individual questions please contact us by e-mail at service@chameleon-pharma.com.