Photo by ALEXANDRE LALLEMAND on Unsplash
Companies from Latin America, Asia, CIS and MENA aiming to enter the EU must obtain Pharma EU GMP for their manufacturing facilities and complete the EU drug registration process. Understanding the regulatory landscape is essential to successfully navigate these requirements. With over 447 million patients, stable market growth with high prices and good margins, the European OTC and Pharma market is the perfect place for expanding your international sales.
Mapping the EU Market Potential
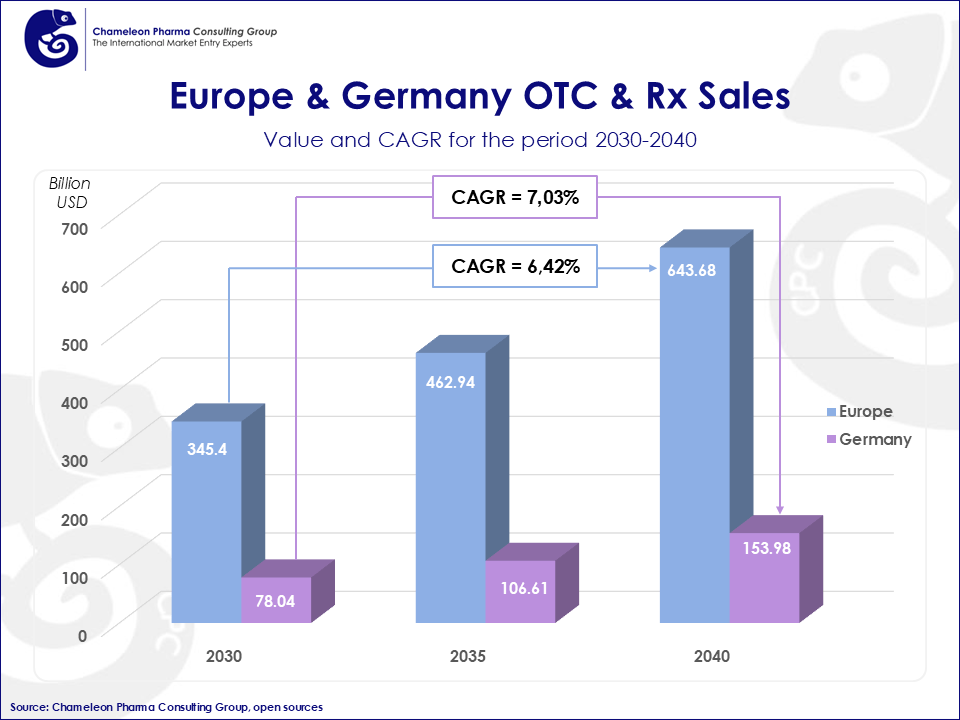
Figure 1: Europe & Germany OTC & Rx Sales projection 2030 till 2040
The EU OTC and Pharma market is projected to grow significantly, with CAGR rates of 6.42% for Europe and 7.03% for Germany between 2030 and 2040. The Consumer Health and prescription drug sales in Europe are expected to exceed $640 billion by 2040. Additionally, 85.5% of sales are driven by prescription drugs, highlighting the importance of entering this market.
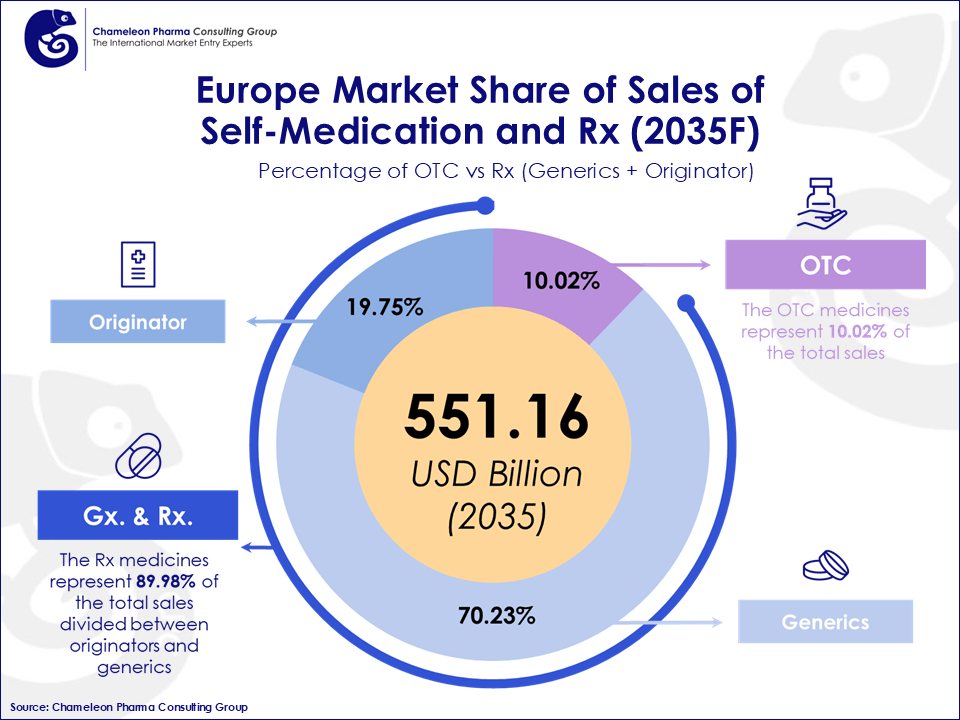
Figure 2: Europe Market Share of Sales of Self-medication and Rx (2035F)
Contrasting to Asia, Latin America and the US, where patients often have to pay for medicines, in Europe hospital stays, surgeries as well as drugs used during treatment are free of charge. This reimbursement for prescription drugs is one of the strongest reasons for attracting foreign companies.
Gain a Competitive Edge: The Benefits of EU-GMP Certification
By obtaining Pharma EU-GMP certificate you have a fast-track registration access in 75+ countries of the world. Your factory and products gain access to not only the European market but also other lucrative regions such as Latin America, EU, Middle East, Australia, US/Canada, Asia in which EU GMP is recognized without extra GMP inspections.
The Advantages of EU-GMP Certification:
- Regulatory Compliance: Having EU-GMP for your factory will ensure compliance with stringent EU requirements like Pharmacovigilance, MDR (Medical Device Regulation), and drug registration guidelines.
- You will be less dependent on your home markets business and can grow international sales
- Market Access: Pharma EU-GMP certification allows you to register and sell your products in Europe, including Germany and the UK, which are key markets.
- Reputation Boost: Pharma EU-GMP certification enhances your credibility and reputation globally, making it easier to secure partnerships and contracts.
Steps to Obtain EU-GMP Certification
The first and most important step is the Pre-EU GMP Audit & Dossier check: Conduct a regulatory GAP analysis to identify bottlenecks and ensure compliance with EU Pharma GMP standards. CPC as a one-stop-shop regulatory service provider can assist you and your company throughout this process. If you want to learn more about each step of how to obtain your Pharma EU GMP, please contact us at service@chameleon-pharma.com to get the second part of this article.
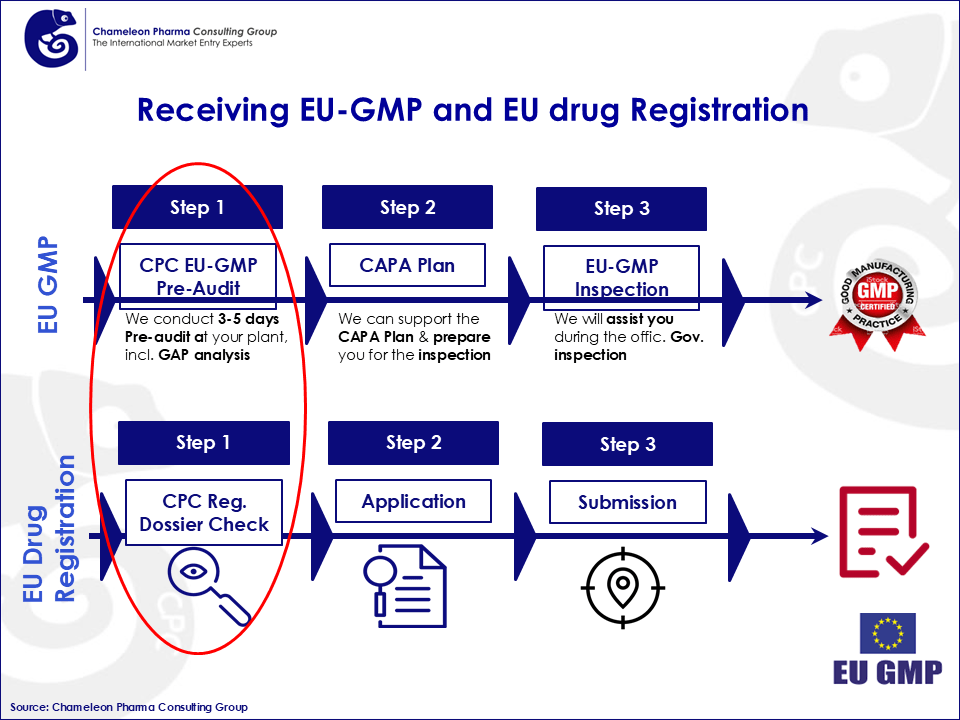
Figure 2: Receiving EU-GMP and EU drug Registration
Accelerate Your Market Entry with CPC’s Regulatory Expertise
At CPC, we specialize in guiding companies through this process. Our systematic approach includes:
- Regulatory dossier checks to ensure compliance with EU drug registration guidelines.
- Preparation for EU-GMP inspections, leveraging our extensive network of inspectors.
- 99% success rate in obtaining Pharma EU-GMP certification.
Why Choose CPC?
- Experience: We have a proven track record in Latin America and Asia, understanding the unique challenges faced by companies in these regions.
- Speed: Our streamlined process ensures quick certification.
- Network: Our connections with EU inspectors give you a competitive edge.
- With our CPC Pre-Audit you have 90% of the EU-GMP preparation
done.
The EU Consumer Health and Rx market offers unparalleled opportunities for companies from Latin America, Asia, and MENA. With CPC’s expertise, you can navigate the complex regulatory landscape and unlock the potential of this lucrative market. Let us support you obtain the Pharma EU-GMP certificate and EU drug registration and take your business to the next level. To find more detailed steps about the process you can join our upcoming expert webinar: Roadmap to receive Pharma EU GMP & EU Drug Registration.
Chameleon Pharma Consulting Group (CPC) has over 20 years of experience in supporting Pharma, OTC, Medical Devices, Phyto, and Aesthetic Medicine companies. Having established own offices & local hubs across Latin America, Europe, Asia, the US/Canada, the Middle East, and the CEE/CIS regions is another advantage of CPC. With this local network and expertise gained from 300+ international projects and a team of 25 experts we offer our clients:
- Business Development, M&A, and Due Diligence
- Market Entry & Expansion: Systematic product and country analysis, market reports
- Strategic Partnering: Identifying local partners, acquisitions, or setting up own offices
- Regulatory & Registration: for drugs, MD, Derma, Aesthetic Medicine, etc.
- Market Authorization & Compliance: Holding MAs, conducting pharmacovigilance
- Quality & Certification: GMP certification, pre-GMP audits
Contact us today for your individual request at service@chameleon-pharma.com!




