Photo by Francisco Kemeny on Unsplash
Get The Important Insights!
The pharma market in Chile has been experiencing a consistent development and growth during the last years. According to CPC experts, the demand for over–the–counter and Rx drugs is expected to drive sales in the Chilean pharma market to USD 5,27 bln by 2035 (at ex-factory price), making it an attractive investment opportunity. Similarly, the expanding network of pharmacies in Chile is creating a receptive environment for the introduction of treatments, such as bioequivalents, generics, and innovations.
Insights into Rx and OTC Drug Registration in Chile
To enter the Chilean pharma market, each new product must go through a comprehensive registration process under the strict guidance of the local Public Health Institute (ISP). The ISP is accredited by the Pan-American Health Organization (PAHO), ensuring that the sanitary registration in Chile adheres to the highest quality and safety standards of the Pan-American regulatory system.
The regular drug registration process for pharma and over-the-counter products generally takes around 7 months to complete, provided all required documentation is submitted on time and all applicable fees are paid. In certain circumstances, the registration may be revised, revoked, or halted by the ISP or by the registration holder.
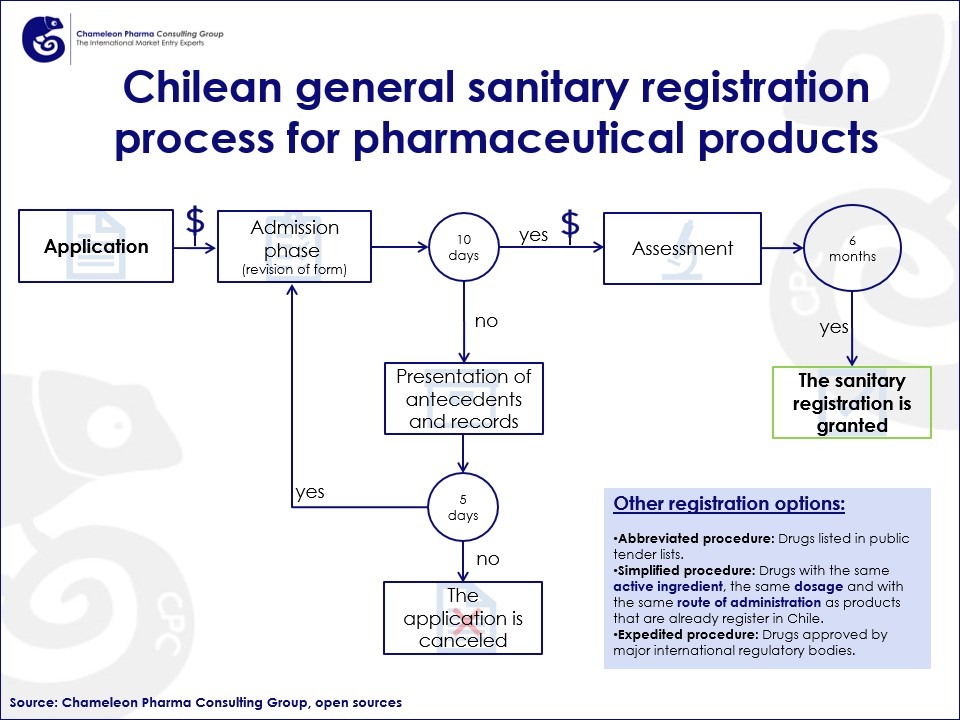
Figure 1. Standard sanitary registration process for OTC and pharma drugs in Chile
Apart from the regular registration process, there are 3 other special registration procedures that can be completed within 5 months of receiving the application and background information.
These special registration procedures are:
- Abbreviated procedure: Drugs listed in public tender lists. The total duration of the procedure is 4 months.
- Simplified procedure: Drugs with the same active ingredient, the same dosage and with the route of administration as products that are already register in Chile. The total duration of the procedure is 5 months.
- Expedited procedure: Drugs approved by major international regulatory bodies, the total duration of the procedure is 3 months.
The expedited procedure, the fastest special registration procedure in Chile, is available for Rx and OTC products that have already been approved by major international regulatory bodies (i.e. FDA or EMEA) as well as WHO, PHO and PIC/S. This streamlined process offers a compelling route for bringing innovative drugs from other markets to the Chilean pharma landscape.
Since the domestic production in Chile is predominantly focused on generic Rx and OTC drugs, most of the innovative products are imported. In fact, Germany and India account for 28% of the total purchases of OTC and Rx drugs made by the National Supply Center (CENABAST). Given that experts calculate the participation of CENABAST to be a substantial 70% of the Chilean pharma, particularly public tender, market, the participation of Germany and India can be considered significant.
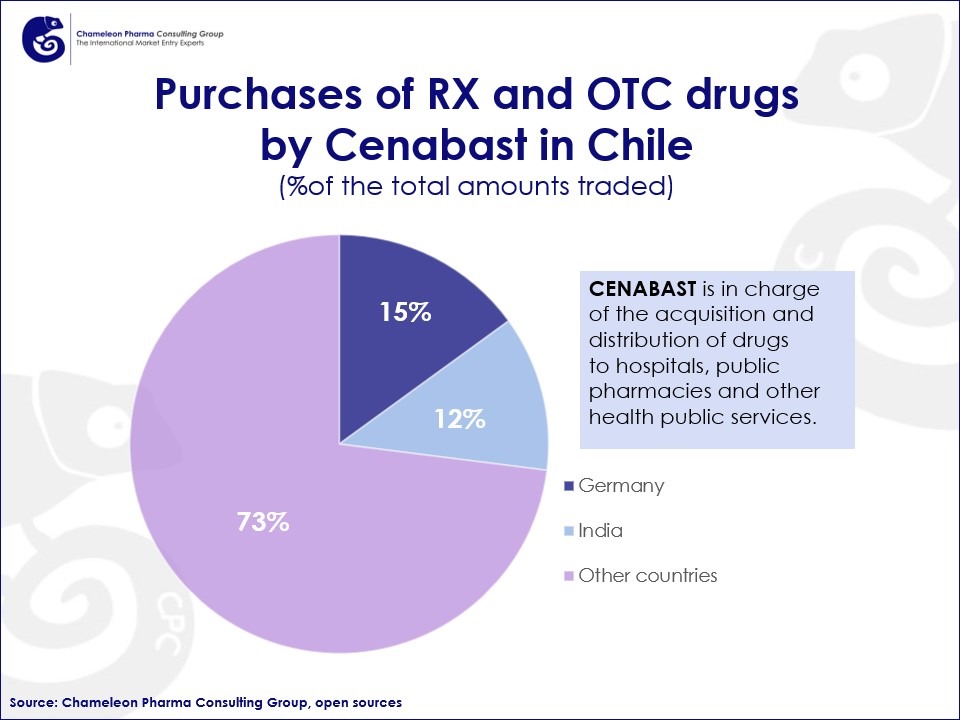
Figure 2. Purchases of RX and OTC drugs by CENABAST in Chile
Over the past few years, imports of personal use medicines have increased by 148%, with only 5% of applications being denied. This suggests that there is room for the introduction of new drugs in the Chilean pharma market, particularly in the segments of nervous system diseases, respiratory diseases, and skin diseases, as these have emerged as the most prevalent diagnoses in the recent period.
Chameleon Pharma Consulting Group, with over two decades of expertise, has been instrumental in assisting and guiding companies seeking to enter or expand their reach into global pharma markets. With a team of 22 experts strategically positioned across the globe, CPC has successfully completed over 300 projects across several continents.