Photo by lena serditova on Canva Pro
Germany’s MedTech market is projected to reach approximately USD43.5 billion by 2030. The German all-together with the European Medical Device markets represent some of the largest and most well-regulated healthcare sectors worldwide. Their size, comprehensive reimbursement systems, and high demand for innovative medical devices (MDs) make them attractive to international companies seeking expansion.
The German and European MedTech Market: A Hub of Opportunity
With exports accounting for 65%, the German MedTech industry employs over 250,000 people, reflecting its critical role in the German economy. Nearly 93% of German Medical Device companies are SMEs, emphasizing the sector’s diversity and innovation potential. On the other hand, the EU medical technology industry employs directly more than 880,000 people.
Europe, as a whole, is a major player, with its Medical Device market valued at approximately €160 billion, representing 27.6% of the global market. Its Medical Device market is projected to grow at a CAGR of 4.5% by 2030.
Despite its well-established presence, Germany and the EU continuously demand new, high-quality medical technologies due to aging populations, rising chronic disease cases, and healthcare digitalization. International MedTech companies with innovative solutions can benefit from this demand and gain a competitive advantage in a high-value market.
The German Reimbursement System: A Key Advantage for Market Entry
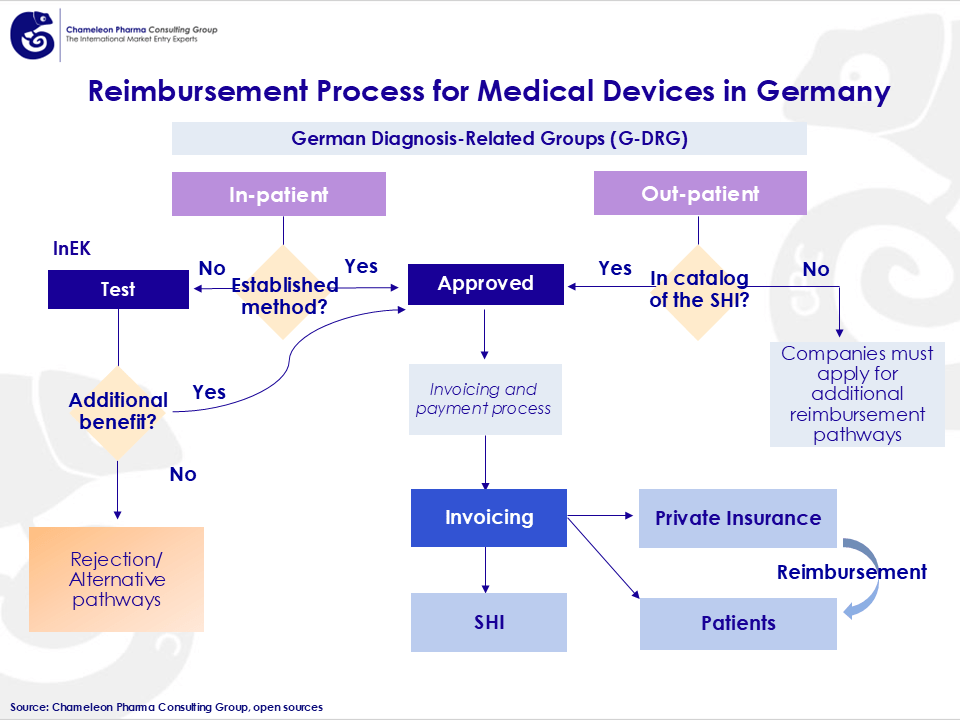
Figure 1: Reimbursement Process for Medical Devices in Germany
One of Germany’s most attractive features for Medical Device companies is its comprehensive reimbursement system. Roughly 90% of the German population is covered by statutory health insurance (GKV), ensuring broad access to reimbursed medical devices.
Key aspects include:
- Hospital Reimbursement (DRG System): Hospitals receive fixed payments for treatments, including associated medical devices.
- Outpatient Reimbursement (EBM and GOÄ): Medical devices used in outpatient care can be reimbursed through standardized pricing models.
- Health Insurance Approval Pathways: Innovative devices can be introduced through the Innovation Fund and selective contracts with insurers.
For international companies, securing reimbursement approval is essential to achieving widespread adoption and financial success.
Rising Demand for Medical Devices in Germany and Europe
The German and European healthcare sectors are experiencing a growing and unmet demand for medical devices, driven by:
- Aging Population: By 2050, over 30% of the EU population will be aged 65+, increasing demand for orthopedic implants, diagnostic imaging, and remote monitoring devices.
- Chronic Disease Growth: Conditions such as diabetes and cardiovascular diseases are rising, necessitating advanced treatment technologies.
- Digital Health & AI Integration: Germany’s Digital Health Act (DVG) allows digital medical applications (DiGA) to be reimbursed, providing opportunities for AI-driven and connected devices.
This sustained demand makes Germany and the EU prime markets for MedTech innovation.
CPC Supports Market Entry and EU GMP Compliance
Expanding into the German and European MedTech market requires navigating regulatory requirements such as EU MDR (Medical Device Regulation) and achieving Good Manufacturing Practices (GMP) compliance. CPC provides tailored support in:
- Regulatory Strategy & EU MDR Compliance/EU GMP: Ensuring products meet EU standards and approval.
- Market Entry & Distribution Partnerships for Medical Devices, Pharma, OTC and Aesthetic Medicine: Helping companies connect with key stakeholders and distributors.
- Reimbursement Pathway Navigation: Assisting with applications for statutory health insurance coverage.
Germany and the EU offer a large, well-funded, and innovation-driven MedTech market. However, navigating compliance challenges requires expertise. With the right approach, international MedTech firms can successfully establish themselves in Germany and the EU, tapping into one of the world’s most advanced healthcare markets. Stay tuned for our next two articles where we unveil the roadmap to achieving EU GMP, or join our free Expert Webinar: Roadmap to receive Pharma EU GMP & EU Drug Registration. Learn more about the webinar here.
Chameleon Pharma Consulting Group (CPC) has over 20 years of experience in supporting Pharma, OTC, Medical Devices, Phyto, and Aesthetic Medicine companies. Having established own offices & local hubs across Latin America, Europe, Asia, the US/Canada, the Middle East, and the CEE/CIS regions is another advantage of CPC. With this local network and expertise gained from 300+ international projects and a team of 25 experts we offer our clients:
- Business Development, M&A, and Due Diligence
- Market Entry & Expansion: Systematic product and country analysis, market reports
- Strategic Partnering: Identifying local partners, acquisitions, or setting up own offices
- Regulatory & Registration: for drugs, MD, Derma, Aesthetic Medicine, etc.
- Market Authorization & Compliance: Holding MAs, conducting pharmacovigilance
- Quality & Certification: GMP certification, pre-GMP audits
Contact us today for your individual request at service@chameleon-pharma.com!




