The global medical device market is a constant source of innovation, fueled by steady growth and ever-evolving opportunities. It is projected to reach USD 795 bln by 2030 at a CAGR of 5,2%. When introducing a new medical device to the market, a thorough understanding of the regulatory institutions’ processes and requirements is key to success. This article aims to give you important insights into the registration pathway in the EU and Latin America
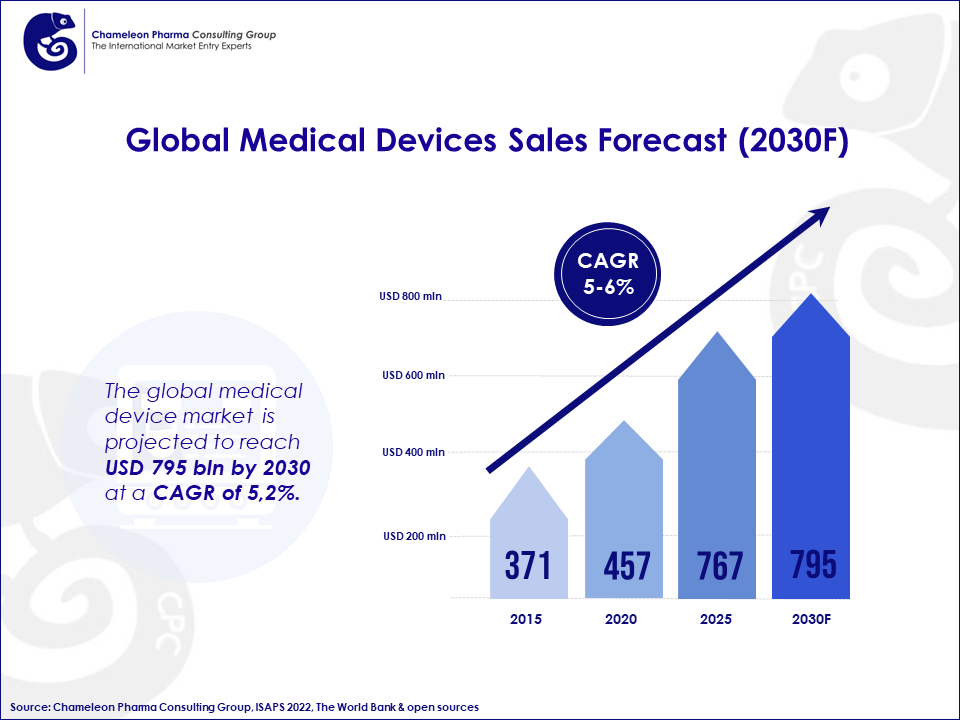
Figure 1. Global Medical Devices Sales Forecast (2030)
Regulatory Landscape
In Latin America, the medical device registration process lacks harmonization among countries. Each country has its own regulatory authority overseeing the approval process, i.e. COFEPRIS in Mexico, INVIMA in Colombia, and ANVISA in Brazil.
In Europe on the other hand, the Medical Device Regulation (MDR) was introduced to standardize procedures and enhance safety across the union. The MDR regulation is an update to the previous Medical Device Directive (MDD), aiming to enhance the safety and quality of medical devices for patients and consumers. The regulation will apply to all medical devices and in-vitro diagnostic medical devices (IVDs) sold or used within the EU, regardless of where they were manufactured. It came into force on May 26, 2021, but the EU updated the dates of application to the following:
- May 26, 2024 – End of transition period for legacy devices that do not meet the conditions for application to the new transition periods
- May 26, 2026 – End of derogation for class III custom-made implantable devices
- December 31, 2027 – End of transition period for class III and IIIb implantable devices
- December 31, 2028 – End of transition period for other class IIb, IIa, class I sterile/measuring devices, devices requiring notified body involvement for the first time under MDR
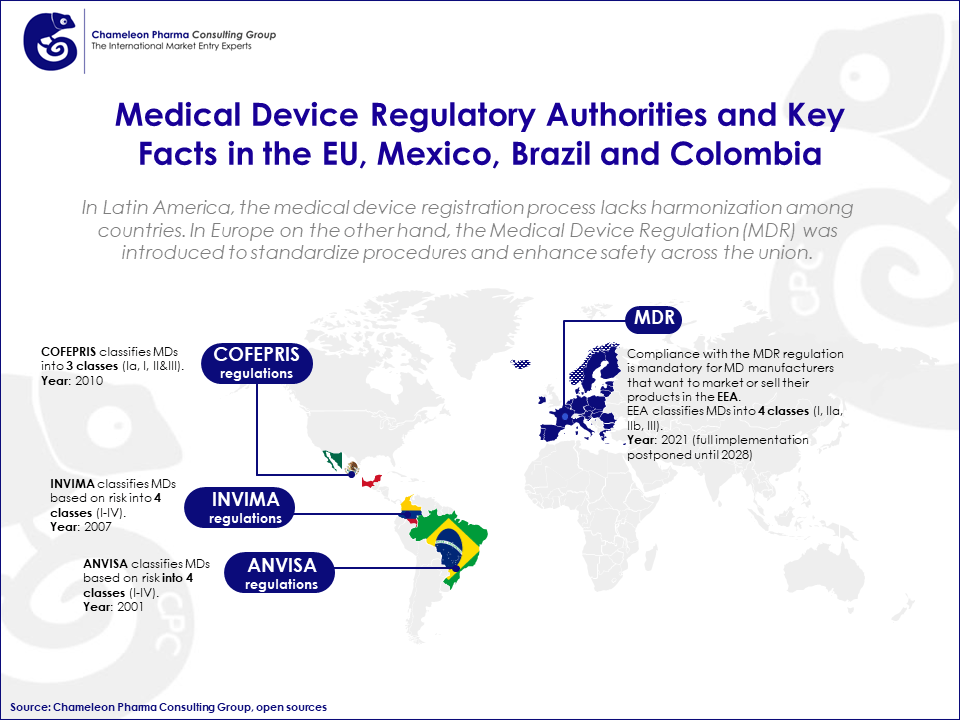
Figure 2. Medical Device Regulatory Authorities and Key Facts in the EU, Mexico, Brazil and Colombia
Classification, Costs and Timeline
Estimated medical device registration costs vary from country to country, often depending on the chosen procedures and local regulatory bodies. These costs are based on publicly available information and might not reflect the final costs, varying from case to case.
Costs within the EU can vary significantly depending on the chosen conformity assessment route (self-declaration vs. notified body involvement) and the complexity and risk class of the device. They can range between EUR 5.000 and 50.000. Additionally, the manufacturers are expected to pay the notified bodies fees up to EUR 50.000, depending on the product class and complexity.
In Mexico, the registration fees for medical devices and in-vitro diagnostic (IVD) vary based on their classification and are stipulated by the Federal Commission for Protection against Sanitary Risks (COFEPRIS). However, it is necessary to take into account additional costs coming with the registration process.
Similarly, Colombia’s registration fees are indicated by the National Institute of Surveillance of Pharmaceuticals and Food (INVIMA), are based on the complexity of the devices and are often accompanies by significant additional costs.
The complexity of the Brazilian ANVISA’s process makes it difficult to provide specific cost estimates for each class. The official fees and time can be significantly altered in case a company opts to file a lawsuit against Brazilian Association of the Health Technology Industry (ABIMED) to force GMP inspection within 6-8 months.
It is important to keep in mind the pre- and post-marketing activity costs, including GMP fees, product certifications, in-country representation, translation of documents, as well as pharmacovigilance, variations, renewal and post-market audits.
Overall, the EU MDR presents the highest registration complexity compared to the regulatory frameworks in Mexico and Colombia. The rigorous requirements, extensive involvement of Notified Bodies, and stringent post-market surveillance contribute to the complexity. The Brazilian ANVISA also presents significant challenges to new market entrants. In contrast, while COFEPRIS and INVIMA also have thorough regulatory processes, the requirements are generally less stringent, particularly for lower-risk devices. It is important to note that owning an EU MD registration will significantly facilitate the local registration process in Latin America.
Companies looking to market medical devices in these regions must prepare for varying levels of regulatory complexity and ensure compliance with each jurisdiction’s specific requirements. Partnering with a local regulatory expert can significantly facilitate this process by avoiding typical mistakes.
Chameleon Pharma Consulting Group specializes in internationalization, market entry and regulatory projects in the areas of consumer health, pharma, food supplements, medical devices, and cosmetics in the Middle East, as well as Latin America, Asia, the US, Europe, and CIS. We have completed over 300 international market entry projects, offering:
- Systematic Country and Product analysis (portfolio analysis),
- Systematic Local Partner Identification,
- Registration and Regulatory Affairs,
- Digital Transformation Services (strategy consulting, e-learning, social media analysis, SEO optimization and other).
For your individual questions please contact us by e-mail at service@chameleon-pharma.com.