OTC and Pharma Regulatory and Registration
Over the past 20 years, we have gained extensive regulatory and registration experience in Latin America, Asia, Russia/CIS, the US, Middle East, and Europe. We worked in various segments such as Pharma/Rx, Consumer health, MD, FS, Cosmetics, etc.
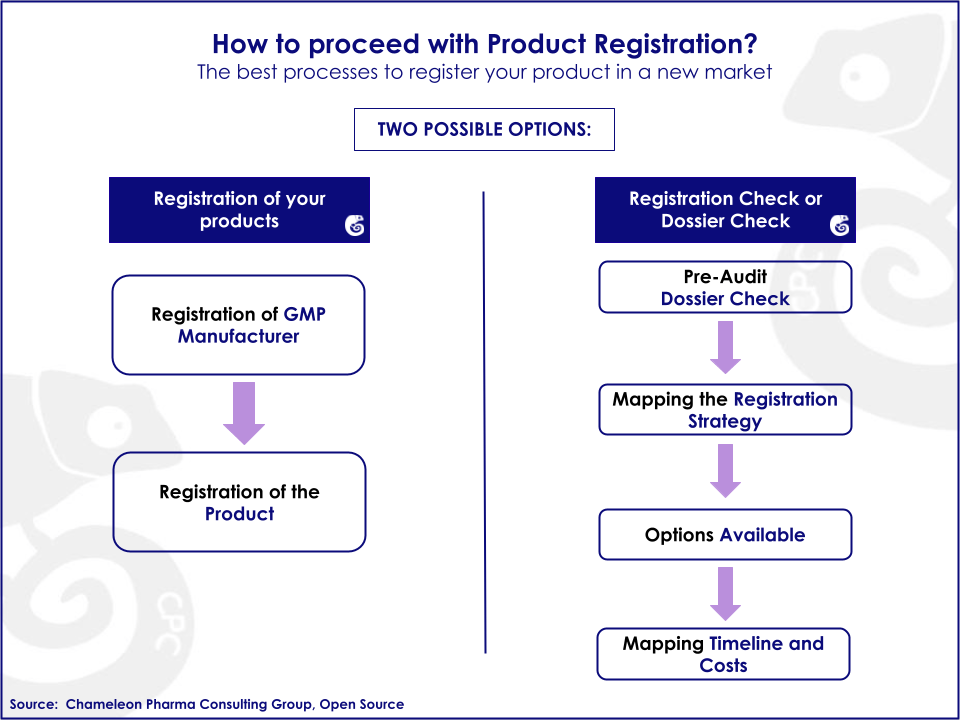
Our Regulatory services overview:
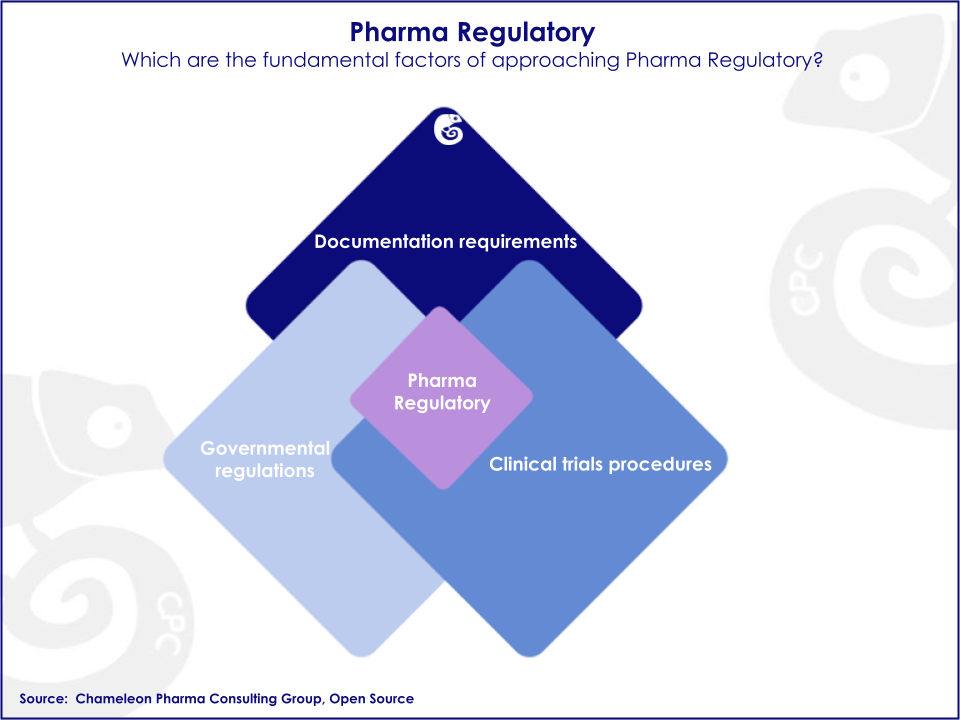
Importance of Pharma Regulatory Processes
Since the Pharma Regulatory and registration process is linked to the development of new products, this process represents the first essential step to successfully enter a new market. Therefore, the first step in ensuring the success of pharmaceutical companies depends on gaining the marketing authorization approval from the regulatory body for their Pharma and OTC products. Pharma Regulatory, in general, is a decisive element that enables the commercialization of new healthcare products. Procedures can vary widely between countries, as requirements are country-specific. Regulatory professionals and local regulatory know-how take on an important role right from the beginning of the development of pharmaceuticals and throughout the lifecycle of the product.
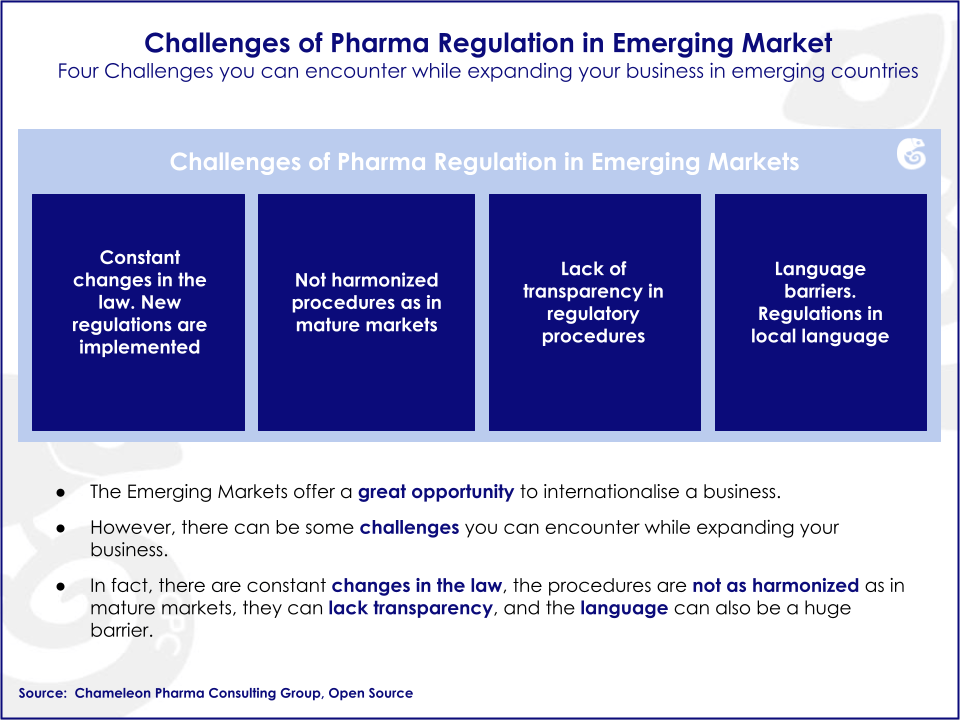
Pharma Regulatory in Emerging Markets
Due to the fast growth of the pharma and OTC industry in Emerging Markets, there is a need for rapid adaptation. The regulatory conditions of pharma emerging markets like Russia, Brazil and other markets are constantly being updated, as improvements are still needed. For example, during the lifecycle of a single product, Emerging Markets can experience regulatory updates and significant changes in the registration processes. Additionally, regulatory procedures and communication with local authorities is not always clear and straightforward in these markets.
CPC offers the most up-to-date information on regulatory changes and on local networks in the global pharma industry. We will gladly assist you in registering your pharma products, and guaranteeing your company a speedy market entry.
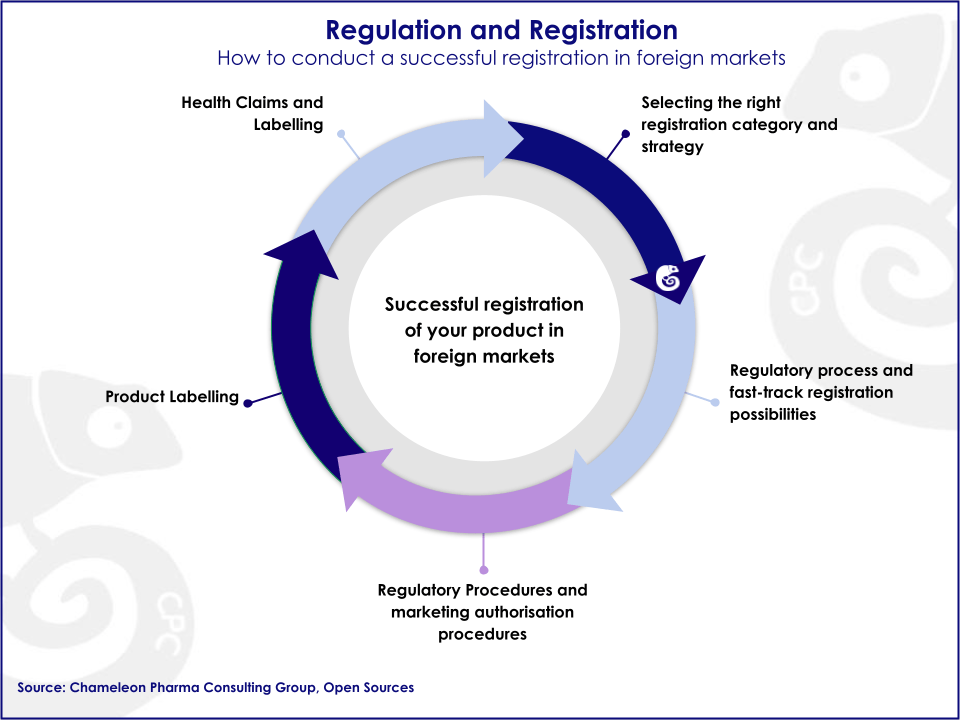
Regulations governing the healthcare industry have been designated by governments to protect public health, and before companies can pursue success in the pharma business, they will have to meet these regulatory requirements. Regulations are diverse and country specific, and therefore, a comprehensive international Regulatory strategy is essential in order to successfully launch new products onto the market.
Today´s dynamic Pharma Regulatory environment demands high expertise and knowledgeable partners who can help you ease the way to success. Chameleon Pharma Consulting has developed a method to help you answer all your regulatory questions and launch your products onto the market. We especially support you in increasing your pharma and consumer health product portfolio in Emerging Markets.
Pharma Regulations represent a process in the area of public health which is developed by governments in order to protect consumers´ health. All pharma and OTC companies need to meet these specific governmental requirements to get new products approved. Pharma Regulations are involved in every stage of the drug development process. The development of new OTC and Rx products constantly brings with it changes in governmental regulations, documentation requirements, and clinical trial procedures which should be addressed from an international perspective. The pharma market is complex and not only is it continually changing but also the corresponding regulations. Due to these on-going changing rules on Pharma Regulatory around the globe, it is advised to have the latest information on current modifications and upcoming changes.
We are the experts
All of our Consultants have up-to-date knowledge about current legislations, regulatory requirements and registration processes in Europe and Emerging Markets, as well as a fundamental understanding of how to interpret these regulations correctly. Mature markets, such as Europe, Japan and the US, have well-established healthcare systems and are urging for synchronization of procedures; for instance, they have developed a Common Technical Document which aims to standardize technical requirements for the registration of new drugs.
If you would like to continue your journey through Mature & Emerging Markets (specifically Brazil, Russia, and Mexico) in regards to Regulatory affairs and Registrations, please contact us.
Some examples of our Regulatory knowledge:
The Registration process of drugs in Brazil has to be aligned to the regulatory scheme of the National Agency of Sanitary Surveillance (ANVISA). ANVISA was founded in 1999 and is responsible for the regulation of healthcare related products. ANVISA approves Product Registration Dossiers (PRD) and is responsible for the regulation of several public organizations in the healthcare sector. Special attention should be paid to the submission of PRDs to the regulatory agency as inconsistencies often lead to time-consuming and costly registration procedures, which may jeopardize the market entry of new products.
The approval of new products by ANVISA is based on the review of the Product Registration Dossier (PRD). The PRD is a compilation of legal and technical documents which are specific for each type of product. The PRD is submitted to ANVISA which consequently approves the request if the documents support efficacy and safety of the product. Once the approval is obtained, prices are set and product importation can take place.
Challenges to get regulatory approval in Brazil:
- Process is complex and confusing at times
- Language barriers
- Lack of local knowledge (often delays the approval of the product)
It is important to have a reliable partner who can help you navigate this procedure.
We are happy to assist you during the entire registration process, beginning with the application for your product, until the final launch. All our CPC experts have a minimum of 20 years’ experience in the global pharma industry, including the Brazilian market.
The registration process in Russia can be quite complicated and sometimes problematic for foreign manufacturers. Product testing is a fundamental precondition of getting new products launched. First, there are preclinical studies needed to collect data for new treatments. These are required in order to create a registration dossier for clinical trials in the Russian Federation. As its own national standardized tool, Russia, as other European countries, uses this method for determining product safety and efficiency.
Pharma Regulatory in Russia is carried out by the National Centre of Pharmaceutical Products Expertise (FGU). The process of Pharma Regulatory goes through several institutes and departments. Additionally, the Pharma regulatory environment in Russia has changed significantly over the last years and more and more changes are expected to occur in the following years. As a result, Rx and OTC companies should be aware of the up-to-date regulations and be able to adapt, assuring them an edge over companies without this capability.
Furthermore, as a result of the Russia Pharma 2020 Strategy which aims to modernize the pharmaceutical industry in Russia, the government is expecting to substitute 50% of all generic drugs with domestic alternatives by 2017. The Russia Pharma2020 Strategy also includes a plan for manufacturing 50 % of all drugs domestically by 2020, which will make it more difficult for foreign companies to complete the registration process.
Challenges to get regulatory approval in Russia:
- Process is complex and confusing
- Language barriers
- Governmental regulations are changing
- Country specific requirements are difficult to understand
It is important to have a reliable partner who can help you comply with the current Russian regulations.
Our Experts can support you and your company successfully throughout your registration process and market entry into the Russian market.
In February 2005, COFEPRIS implemented renewals of drug registrations in the Mexican Pharma Market. Rx and OTC products in Mexico are governed by the Commission for the Protection against Sanitary Risk (COFEPRIS) and must be approved before they are commercialized in the country.
The assessment of the application is carried out by COFEPRIS regulatory experts. At times, regulatory experts fail to understand the nature of documents coming from Europe and other countries, which may lead to delays in the approval process. Therefore, having experience when dealing with imported RX and OTC products, as well as knowledge of local requirements, are essential factors for companies striving to get their products into the Mexican market. Further, COFEPRIS has established new registration procedures for Fast Track registration of Consumer Health and Pharma products:
- Private organizations have been authorized by COFEPRIS to pre-review PRD
- COFEPRIS has recognized GMPs from specific countries
- COFEPRIS has signed equivalency agreements to expedite registration of products already registered in specific countries.
CPC has a long-standing network acquired through engagement with several projects in Mexico. We are happy to help you with your international strategy and are here to make your market entry easier into the Mexican Pharma Market.
Our Areas of expertise:









Are you interested in any other country?
If your areas of interest include other countries compared to the markets above, feel free to contact us through the button below!