Cityscape of Cairo City by givagaphotos from Canva
The Egyptian consumer health and pharma market has experienced significant shifts over the past few years, driven by diverse factors such as regulatory reforms, innovations in healthcare, and changes in patient and consumer demand. Egypt remains the second-largest market in the Middle East and North Africa (MENA) region, and the projected value of the OTC and Rx market is estimated to reach approximately USD 21 bln by 2035 at a CAGR of 14%.
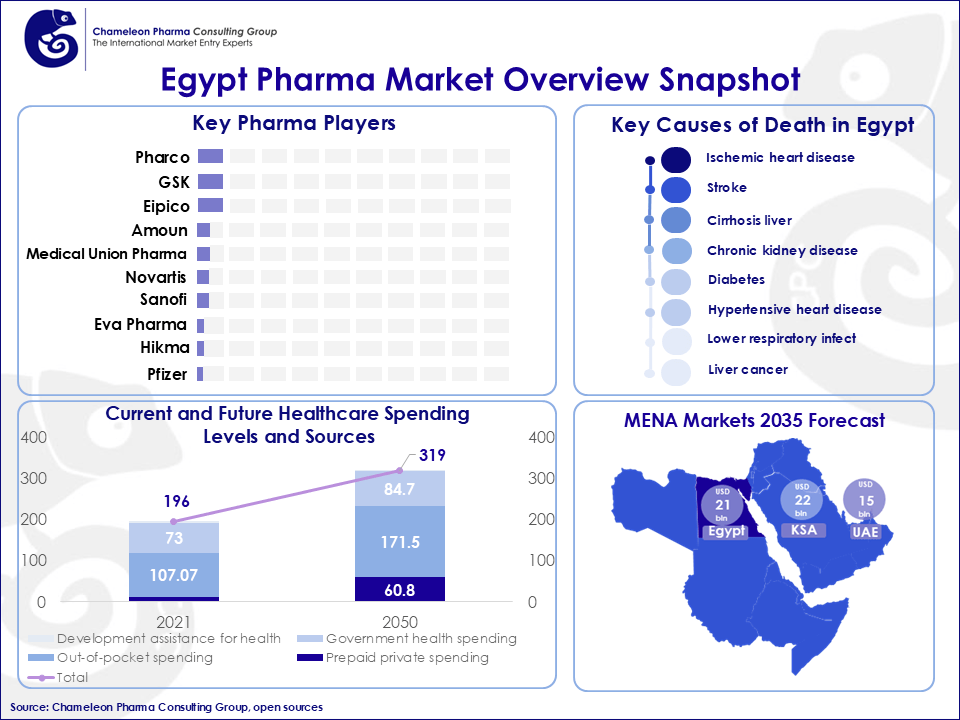
Figure 1. Egypt Pharma Market Overview Snapshot
Overview and Trends of the Egyptian Self-Medication & Pharma Market
The hospital channel, which represents 23% of Egypt’s total consumer health and pharma sales, has been experiencing active growth in the past years. However, the retail sector, particularly in urban areas, is the main driver of market expansion. In the retail sector:
- The systemic antibacterial therapeutic area dominates with an 11% market share.
- Antihypertensives are the fastest-growing category, with a 1.9% .
Leading corporations such as Hikma Pharma are thriving, with products like Tusskan, Omeral, and Megamox recording exceptional growth rates. Hikma’s rapid growth reflects:
- Evolving consumer demand for respiratory, gastrointestinal, and antibiotic treatments.
- A larger trend towards specialization and innovation in healthcare.
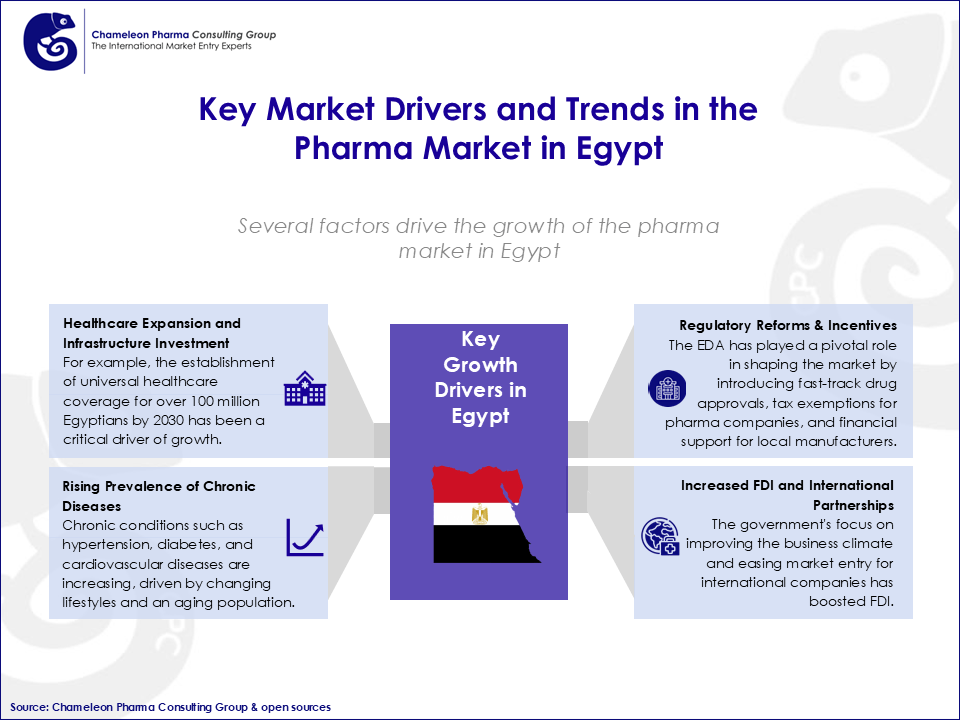
Figure 2. Key Market Drivers and Trends in the Pharma Market in Egypt
Regulatory and Policy Shifts in Egypt
Regulatory reforms spearheaded by the Egyptian Drug Authority (EDA) have catalyzed the market’s expansion. The EDA has introduced flexible regulations, including fast-track drug approvals and emergency use authorizations, which were instrumental during the COVID-19 pandemic. Egypt became the first country in Africa and the Middle East to provide the COVID-19 antiviral Molnupiravir in 2022, significantly reducing hospitalization rates.
These regulatory advancements have not only enhanced patient access to essential medicines but also elevated Egypt’s standing in the global healthcare arena. The World Health Organization (WHO) recognized Egypt’s EDA with a ‘Maturity Level 3’ designation in vaccine production, marking a significant milestone in the country’s healthcare capabilities.
Similar to Saudi Arabia and the UAE, the EDA’s reforms also align with Egypt’s Vision 2030, which aims to:
- Overhaul the healthcare system.
- Achieve universal health coverage.
- Improve infrastructure.
- Enhance access to innovative treatments.
Local production incentives, such as tax exemptions for new facilities and financial assistance for local manufacturers, have attracted significant investment from global players entering Egypt. Companies like EIPICO are constructing a biologicals and biosimilars plant, an investment that will further strengthen Egypt’s position as a pharma manufacturing hub.
Future Prospects
Looking ahead, Egypt’s OTC and pharma market is set to become even more competitive and globally connected. As the market continues to expand, Egypt’s commitment to digital healthcare transformation will also play a crucial role. Increased digitization, including telemedicine and electronic health records, will enable better patient outcomes, while fostering new opportunities for pharma companies to integrate advanced healthcare solutions.
Overall, the Egyptian pharma market stands on solid ground, upheld by strong growth drivers, regulatory reforms, and expanding healthcare infrastructure. The sector offers promising opportunities for both local and international players, poised to thrive as the country continues its transformation into a regional healthcare hub.
Chameleon Pharma Consulting Group specializes in internationalization, market entry and regulatory projects in the areas of consumer health, pharma, food supplements, medical devices, and cosmetics in the Middle East, as well as Latin America, Asia, the US, Europe, and CIS. We have completed over 300 international market entry projects, offering:
- Systematic Country and Product analysis (portfolio analysis),
- Systematic Local Partner Identification,
- Registration and Regulatory Affairs,
- M&A Projects and Identification of Fresh Acquisition Targets
- Digital Transformation Services (strategy consulting, e-learning, social media analysis, SEO optimization and other).
For your individual questions please contact us by e-mail at service@chameleon-pharma.com.




