Image by tawanlubfah from Getty Images Pro from Canva
Drug regulation plays a crucial role in supporting Thailand’s rapidly growing pharma sector, which is ranked as the second largest consumer health and pharma market in , with pharma sales projected to reach USD 14.1 billion in year 2040 at a compound annual growth rate (CAGR) of 8.31%.
The country’s healthcare system is overseen by the Thai Food and Drug Administration (FDA), the main regulatory authority responsible for drug and medical devices approval, classification, and monitoring. It collaborates with international bodies like the WHO and ASEAN to maintain global standards.
The process in applying for OTC & Rx drug approval is separated into two main parts:
- Drug Facility Licensing,
- Pharmaceutical Product Registration and Approval.
Drug Facility Licensing for pharma products produced in facilities overseas requires GMP clearance from the Thai FDA. After obtaining license for a drug facility or GMP clearance, the next step is registering the product with FDA. Documentations must be prepared in accordance with ASEAN Common Technical Dossier (ACTD) and ICH Common Technical Document (ICH CTD). The drug registration process may take up to 155 days, but the approval period may be extended, depending on the type of product being registered. Figure 1 illustrates an example of OTC & Rx drug registration process.
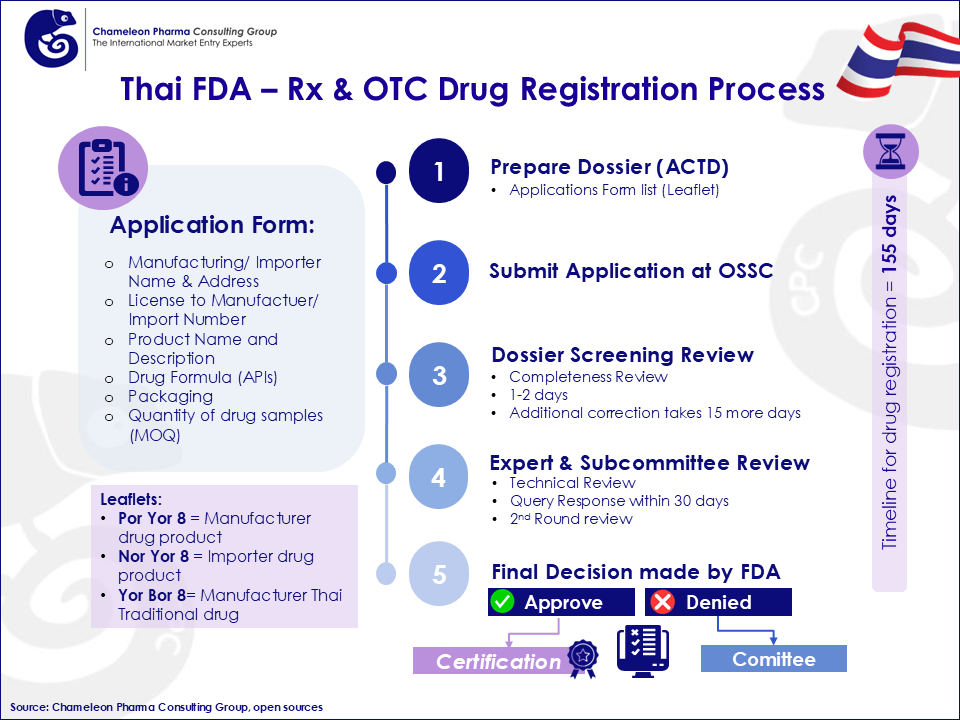
Figure 1: Process Flow in Registering Rx and OTC Drug in Thailand
The Thai National Drug Framework: OTC Drug approval, Pharmacovigilance, and Healthcare Coverage
As for the national drug approval, the process involves rigorous preclinical and clinical trials, along with the Thai FDA’s registration and post-marketing surveillance. Drug monitoring and the National Center were set up in 1983 in which Thailand joined the WHO program for International Drug Monitoring scheme (WHOPIDM) in the following year. Pharmacovigilance ensures the continuous monitoring of drug safety post-approval, focusing on adverse effects.
The framework of drug selection and reimbursement is divided into three levels. It is primarily managed through the National List of Essential Medicines (NLEM), which consists of pharmaceutical reimbursement list for the three public health schemes, namely:
- The Civil Servant Medical Benefit Scheme (CSMBS)
- The Social Security Scheme (SSS)
- The Universal Coverage Scheme (UCS).
The UCS, also known as the “Gold Card”, the Thai UCS has improved access to health care and contributed to Thai economic growth during the past 22 years. Over 99% of Thai citizens are covered by tax-funded healthcare, including support for rare diseases and high-cost treatments, though access to medicines in rural areas remains a challenge.
Essentially, Thailand offers an attractive investment opportunity in the pharma sector, supported by a robust regulatory framework managed by the Thai FDA. The country ensures high drug safety standards through rigorous approval processes and active pharmacovigilance, while integrating international best practices and digital health innovations. Thailand demonstrates a strong commitment to healthcare access and quality.
Despite ongoing challenges such as counterfeit drugs and rural access, the government’s proactive approach to regulatory reform and innovation creates a dynamic and promising market for foreign investors. Regulatory and legal compliance are best managed through partnerships with local companies. Therefore, CPC advises international business developers to prioritize identifying well-suited local partners as a crucial strategic focus.
Chameleon Pharma Consulting Group specializes in internationalization, market entry and regulatory projects in the areas of consumer health, pharma, food supplements, medical devices, and cosmetics in the Latin America, Europe, Asia, US/Canada, Middle East and CEE/CIS. We have completed over 300 international market entry projects, offering:
- Systematic Country and Product analysis (portfolio analysis),
- Systematic Local Partner Identification,
- Registration and Regulatory Affairs,
- Digital Transformation Services (strategy consulting, e-learning, social media analysis, SEO optimization and other).
For your individual questions please contact us by e-mail at service@chameleon-pharma.com.




