Regulatory Guidelines for the Emerging Markets
Regulatory Frameworks differ from country to country and correctly understanding them can be quite challenging, especially in the Emerging Markets.
CPC has developed comprehensive regulatory guidelines for the most important economies of the Emerging Markets. These guidelines will help you to easily understand the current regulatory frameworks with their challenges and help you navigate the maze.
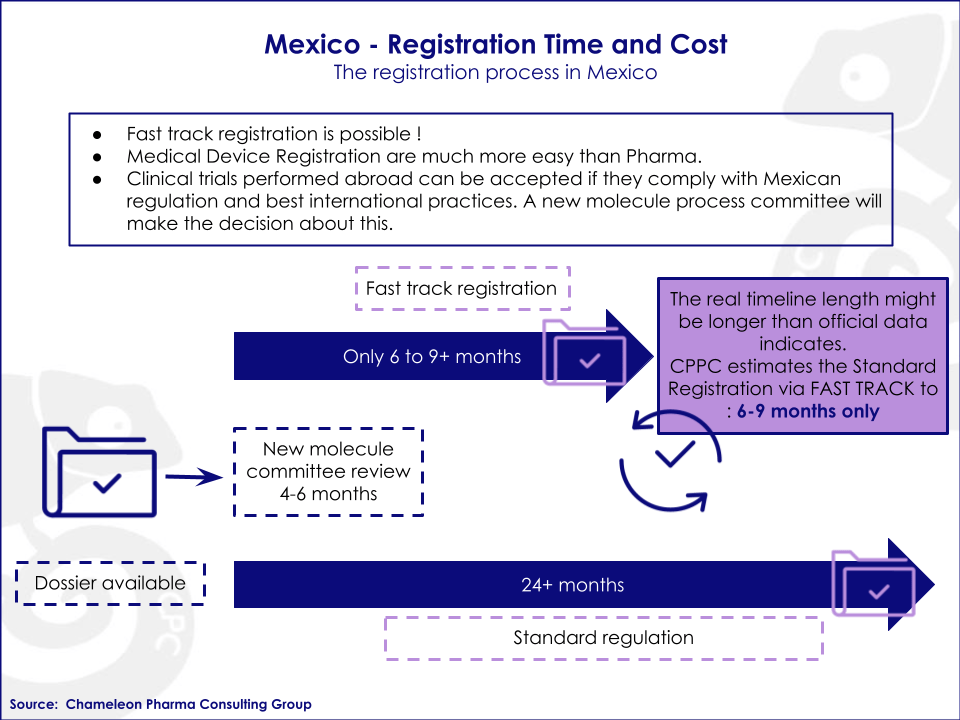
The featured picture is a perfect example of the comprehensive registration process in Mexico.
Features of the Guidelines
- Coverage of the most important economies of the Emerging Markets
- Current regulations for OTC, Rx, Medical Devices, Phytomedicines, Dietary Supplements, Cosmetic and Homeopathic products
- Clear regulatory process to register your products in each country and possible registration short-cuts
- Time, costs and complexity of the registration process
- Applicable regulations during the lifecycle of your product
- Classification of your products in each country
- Post-approval regulations governing the industry
Your Benefits from the Guidelines
- Up-to-date regulations governing the healthcare industry in the Emerging Markets
- Tips to speed up the approval process of your products
- Detailed information on the registration process
- List of documents required for the registration of your products
- Understanding of the regulations to overcome the challenges pharmaceutical companies face in each country
- Classification of OTC, Rx, Medical Devices, Dietary Supplements, Cosmetics, Phytomedicines and Homeopathic
We are ready to respond to your individual regulatory questions and assist through the process of obtaining GMP certificates, product registration, Risk Management reports, design CTD files, variations etc. We are the experts for more than 20 years in Russia, China, Mexico, Brazil and other emerging markets as well as in the European Union.